

REVIEW | Respirar, 2023; 15(1): 60-73 | ISSN 2953-3414 | https://doi.org/10.55720/respirar.15.1.6
RecEIVED:
December 28, 2022
ACCEPTED:
February 1, 2023
Adrián Gallardo
Adrián Dévoli
Cinthia Gigliotti
Eder Zamarrón-López
Orlando R. Pérez-Nieto
Juan Martín Núñez Silveira
Esta revista está bajo una licencia de
Creative Commons Reconocimiento 4.0 Internacional.
High Flow Nasal Cannula in Critically Ill Patients: a Narrative Review
Adrián Gallardo1 , Adrián Dévoli2
, Adrián Dévoli2 , Cinthia Gigliotti2
, Cinthia Gigliotti2 , Eder Zamarrón-López3
, Eder Zamarrón-López3 , Orlando R. Pérez-Nieto4
, Orlando R. Pérez-Nieto4 , Juan Martín Núñez Silveira5
, Juan Martín Núñez Silveira5
1. Sanatorio Clínica Modelo de Morón, Servicio de Kinesiología, Morón, Buenos Aires, Argentina.
2. Sanatorio Clínica Modelo de Morón, Departamento de Clínica Médica Morón, Buenos Aires, Argentina.
3. Hospital General Regional IMSS No. 6, Unidad de Cuidados Intensivos, Tamaulipas, México.
4. Hospital General San Juan del Río, Unidad de Cuidados Intensivos, Querétaro, México.
5. Hospital Italiano de Buenos Aires, Unidad de Terapia Intensiva, Ciudad de Buenos Aires, Argentina.
Corresponding author:
Adrián Gallardo, adriankgallardo@gmail.com
Abstract
High-flow nasal cannula has become one of the main strategies for non-invasive ventilatory support in hypoxemic acute respiratory failure, mainly after the COVID-19 pandemic. However, its use extends beyond this scenario and covers different clinical conditions such as the post-extubation period, post-surgical period, hypercapnic respiratory failure and life support in immunosuppressed, trasplant or cancer patients. Manuscripts that support its application have been widely disseminated and the degree of evidence is high enough to recommend its use. Therefore, it is necessary to highlight its physiological effects such as comfort, precise fraction of inspiratory oxygen, CO2 lavage or optimize end-expiratory lung volume to understand its mechanism of action and improve patients’ outcomes.
The objective of this narrative review is to offer a brief and concise summary of the benefits of applying this therapy in different clinical scenarios without the rigid structure of a systematic review. Based on these lines, the curious reader can expand the scientific evidence that supports the use of the high-flow nasal cannula in each particular scenario.
Keywords: high flow nasal cannula, effects, benefits, pontential risk.
Introduction
Oxygen therapy delivered through high-flow nasal cannula (HFNC) has become popular for the treatment of acute respiratory failure (ARF), mostly during the COVID-19 pandemic. There is enough evidence on the physiological effects and benefits of HFNC in various scenarios and pathologies, so it is important to become familiar with its use, indications and limitations. The aim of this review is to update and summarize many of the available evidence in a simple, straightforward and concise text. Although we have not carried out a systematic search, the information included covers the most relevant aspects of this therapy.
Physiological Effects
Considerable attention has been devoted to understanding the benefits and mechanisms of action of HFNC (figure 1). Unlike low-flow devices, which deliver a variable fraction of inspired oxygen (FiO2) and can have significant leaks, high-flow devices can deliver a more accurate FiO2. Because the increase in FiO2 generates an increase in the inspired pressure of oxygen and the alveolar pressure of oxygen,1,2 its main effect is the improvement in oxygenation, evidenced by the increase in peripheral oxygen saturation (SpO2) and the partial pressure of oxygen in arterial blood (PaO2).
Figure 1.
HFNC equipment and components. Modified from Masclans et al. Medicina Intensiva (2015)
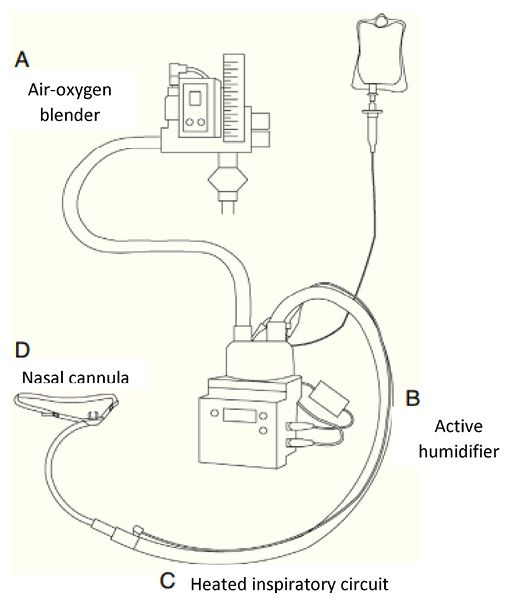
Another effect is the generation of positive end-expiratory pressure (PEEP): nasopharyngeal pressures of 1-4 cmH2O have been reported; if the patient breathes with his mouth open, the pressure will be lower.3,4 Although a recent bench study report a maximum of 1.5 cmH2O with 60L/min.5 The PEEP effect could increase the end-expiratory lung volume (EELV). This effect, besides improving oxygenation, could reduce the collapse of small airways, prevent atelectasis and air trapping.2,6 In addition, HFNC has been shown to improve CO2 removal and reduce ventilatory drive.7 Also, the high flow reduces airway resistance and provides adequate humidity and heat, reducing energy costs.8 For this reason, its use has been considered in chronic obstructive pulmonary disease (COPD).9,10
Some studies have evaluated the work of breathing (WOB). Vargas et al compared WOB with standard oxygen therapy (SO) in 12 patients with ARF and found a reduction of 25% with HFNC at 60L/min.11 Delorme et al measured WOB at 20, 40, 60L/min in patients recovering from ARF and found a 50% flow-dependent decrease at 60L/min.12 Another study measured WOB with HFNC at 40L/min and reported a 25% decrease compared to SO;2 while a later study showed that the magnitude of the effect was flow dependent, with up to a 40% reduction in WOB at 60L/min.6 Furthermore, dynamic compliance was shown to increase.2,6,12 All of the above could explain the reduction in respiratory rate (RR) and minute ventilation (MV) in patients with ARF.2 HFNC contributes to improving homogeneity of alveolar ventilation and reducing stress and strain during spontaneous ventilation, suggesting that it could prevent self-inflicted lung injury (P-SILI).13
Other potential benefits attributed to HFNC are the improvement of secretion clearance and the reduction of upper airway obstructive episodes.1,14 Tiruvoipati et al.15 conducted a crossover trial to compare the impact of post-extubation HFNC versus conventional high-flow face mask; they found no differences in gas exchange, but a better tolerance to HFNC. Rittayamai et al,16 compared the effect of HFNC versus non-rebreathing mask on dyspnea, comfort, and vital signs. They observed less dyspnea, lower RR and heart rate (HR) with HFNC. Finally, Maggiore et al compared HFNC versus Venturi mask for 48h after extubation and found that HFNC produced better oxygenation and lower PaCO2 and RR.1 HFNC effects and benefits are resumed in figure 2.
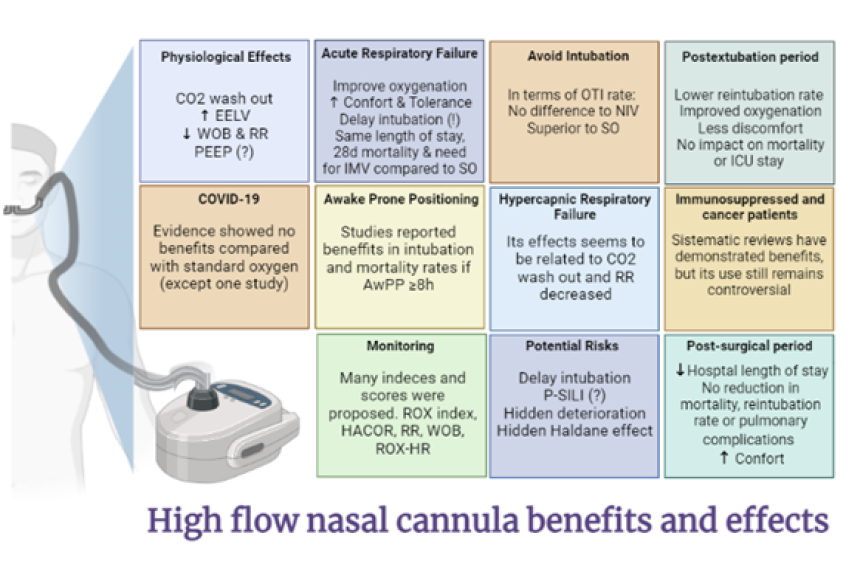
Figure 2.
Benefits and effects of HFNC. CO2: carbon dioxide. EELV: end-expiratory lung volume. WOB: work of breathing. RR: respiratory rate. PEEP: positive end-expiratory pressure. IMV: invasive mechanical ventilation. SO: standard oxygen therapy. OTI: orotracheal intubation. ICU: critical care unit. AwPP: awake prone position. P-SILI: self-induced lung injury
HFNC for acute respiratory failure
There are multiple clinical entities that predispose to the development of hypoxemia. Among the devices used for its treatment, HFNC promotes an improvement in flow-dependent ventilatory parameters17 and can be considered as a first-line treatment for these patients. Because it generates a CPAP-like effect with increased EELV, decreased RR, WOB and right ventricular preload, achieving an hemodynamic pattern improvement,18 its use has been recommended over SO.19 HFNC has traditionally been used in respiratory distress settings due to its ability to improve oxygenation and be more comfortable, although there are controversies about its superiority over SO and the risk of delaying orotracheal intubation (OTI) in different scenarios.20 A meta-analysis showed no significant differences in ICU length of stay, need for invasive mechanical ventilation (IMV), 28-day mortality and SpO2 at the end of oxygen therapy.21 Delayed OTI has been associated with worse clinical outcomes secondary to respiratory muscle fatigue, cardiac dysfunction and multiorganic failure.17 However, Xiaofeng et al.22 demonstrated a lower rate of OTI in patients with HFNC vs. SO; not so with NIV.
HFNC to avoid intubation
The improvement shown by patients with HFNC, compared to those using SO or NIV, has been described previously.23,24 Rochwerg et al.25 show similar results. Furthermore, a RCT found that there were no differences, in terms of OTI rate, in patients with respiratory failure using HFNC or NIV.26 Additionally, a review27 found favorable results for HFNC, observing significant differences in the rate of OTI, compared to SO but not with NIV. Despite the above, not all studies show similar findings. Some authors28 question the disparity in the inclusion criteria of the different studies and the sample size presented in the analysis, which could not reach sufficient power to rule out the null hypothesis and thus yield controversial results.
Postextubation period
HFNC has proven to be successful in specific clinical situations. In a trial that included 310 non-intubated patients with hypoxemic ARF (AHRF), HFNC reduced mortality and the need for intubation, compared with SO or NIV.24 In immunosuppressed patients with ARF, several studies have shown that HFNC may be superior to SO and NIV.29 In surgical patients, HFNC has been used to prevent postoperative ARF and reintubation.30,31
The use of HFNC to prevent extubation failure has been one of the most studied scenarios.32 In an RCT, Maggiore et al1 compared HFNC with Venturi mask in patients with PaO2/FiO2 <300. They found a lower reintubation rate with HFNC, improved oxygenation and less discomfort. Later, Hernández et al. published an RCT comparing HFNC with SO after extubation and found that HFNC decreased reintubation, but no impact on ICU length of stay or mortality was observed.14 However, despite the positive results of this trial, routine application of HFNC is still not recommended.19,33
Subsequently, two RCTs were presented in patients with a high risk of reintubation. In the first, they compared HFNC with NIV after extubation; found no difference in reintubation rates.14 HFNC proved to be not inferior to NIV in preventing extubation failure. The second one compared HFNC with SO and, due to slow recruitment, it was stopped prematurely. There were no significant differences in reintubation or in any relevant outcome.34 The criteria used to determine high risk of reintubation in these studies were based on risk factors associated with outcomes that have not been prospectively validated.33,35 Although these studies may support a role for HFNC after extubation, there are questions about which patients may actually benefit from the therapy.
HFNC and COVID-19
COVID-19 patients present varied symptoms and a small population may present with ARF requiring invasive or non-invasive ventilation. Oxygen therapy is vital, determining in many cases the clinical prognosis. Different works addressed this point using HFNC, SO, NIV or CPAP. CPAP or NIV are sometimes uncomfortable, can lead to decubitus ulcers, require constant monitoring and could significantly increase dead space. In this sense, HFNC was proposed as an option.
At the beginning of the pandemic, Hu et al.36 observed an improvement in oxygenation in 61.9% of patients and that oxygenation indices were closely related to prognosis: a ROX index ([SpO2/FiO2]/RR) >5.55 was significantly associated with therapy success.
Shortly thereafter, a retrospective study37 included 46 patients who were divided into three groups: not-to-intubate decision, without OTI requirements and eventually OTI. They found that patients who ultimately required OTI had a higher RR and a worse PaO2/FiO2 ratio at ICU admission. This subgroup showed a mortality rate of 35%, while HFNC patients survived.
Zhao et al.38 report that NIV is not superior to HFNC in terms of OTI and mortality, although they observed a significant increase in the SpO2/FiO2 index. Another study39 showed the feasibility of using a combined HFNC and CPAP therapy, with favorable results (OTI rate 26.54%, overall mortality 14.15%). Although the study was not designed to evaluate the accuracy of the ROX index, they report that a value of 6,28 showed a sensitivity of 97,6% and a specificity of 51.8%. This value differs from that reported by Ferrer et al.40 who found a value of >5.35 as a predictor of HFNC success.
The study by Garner et al41 evaluated the HFNC failure predictors in patients with COVID-19 and found failure in 76.7% of those who presented a higher SOFA score at admission and at least one comorbidity or history of immunosuppression. Another retrospective study41 with patients who used HFNC or NIV, showed that almost half of the patients who used HFNC subsequently had to use NIV as rescue therapy. Despite the above, the authors conclude that there were no significant differences between groups in terms of duration of therapy, OTI rate or mortality.
To our knowledge, there are few RCTs evaluating the efficacy of HFNC therapy, compared to SO or NIV, in this population. These studies show contradictory results. Teng et al.43 reports that HR and RR were better in the HFNC group after 6h, while the PaO2/FiO2 index was higher at 6h, 24h and 72h. On the other hand, the study by Ospina-Tascón et al.44 included 199 and evaluated the rate of OTI and clinical recovery at day 28. They reported OTI rate of 34.3% for CNAF and 51.0% for SO and a median clinical recovery of 11 days for the HFNC group and 14 days for the SO group. The RECOVERY RS study,45 by contrast, evaluated the OTI rate at 30 days in three treatment arms. The authors report an OTI rate of 41.6% for the SO group, 41.1% for the HFNC group and 33.4% for the CPAP group. Mortality rate at 30 days was: SO 18.8% vs. HFNC 20% and SO 19.2% vs. CPAP 16.7%. Thus, HFNC therapy was not associated with a lower rate of OTI or mortality when compared to SO. This finding coincides with what was reported in a recent case-control study.46 Furthermore, Crimi et al.47 found no benefit from the use of HFNC in patients with mild hypoxemia due to COVID-19.
HFNC and awake prone positioning
Awake prone positioning in patients with ARF and HFNC gained popularity during the COVID-19 pandemic; however, it remains a matter of debate regarding clinical results. A multicenter meta-trial demonstrated a lower need for intubation and better results in patients who remained prone for >8h.48 Meta-analyses and other multicenter studies have shown an association with a lower rate of OTI and decreased mortality,49,50 even in severe ARDS.51 Regarding when to start this position, a higher success rate has been shown if it is used prior to 24h of HFNC use.52
HFNC in hypercapnic respiratory failure
HFNC therapy is widely used in patients with ARF and its benefits have been widely reported; however, the evidence in patients with hypercapnic respiratory failure is limited. The use of HFNC in patients with hypercapnia increased after a reduction in CO2 rebreathing was demonstrated.13,53 Fricke et al. demonstrated that HFNC reduce CO2 levels by flushing dead space in the upper airway by invasive measurement of inspired CO2, ETCO2 by trans-tracheal catheterization, and transcutaneous CO2 in a patient with a tracheostomy tube.54 The application of a flow of 30L/min generated a CPAP effect of 1cm H2O, reduced inspired CO2 from 6 to 3mmHg and transcutaneous CO2 from 68 to 63mmHg, which occurred from the start of therapy, achieving a reduction in minute volume 7.2 to 6.5 L/min. The improvement in ventilation could be explained by the decrease in the anatomical dead space in the conduction airway caused by the high flow of O2. Even a small decrease in dead space can significantly improve ventilation and reduce hypercapnia by increasing alveolar volume and decreasing PaCO2, explained by the following equation:
PaCO2 = k (VCO2 / VA) = k (VCO2 / VE – VD)
where arterial CO2 pressure is equal to CO2 production divided by alveolar ventilation (VA), which can be calculated by subtracting dead space (VD) from minute volume (VE). Subsequent studies on the use of HFNC in patients with COPD, pneumonia, and other causes of hypercapnic respiratory failure have supported these findings55 and a randomized controlled study by Alnajada et al comparing the use of HFNC versus low-flow devices such as initial therapy in patients with hypercapnic respiratory failure may help determine what might be the best approach for these patients.56
Since NIV has been the standard therapy in patients with respiratory failure and hypercapnia, comparative studies have been conducted to test the safety and efficacy of the use of HFNC in this type of respiratory failure. In a study that evaluated the use of HFNC versus NIV for the treatment of moderate hypercapnia, the authors found no significant differences in the rate of OTI at 48h; but when evaluated at 28 days, patients with HFNC had higher values.57 Likewise, these patients showed higher mortality at 28 days and longer stay in the ICU. In a similar group, Nam et al.58 found that HFNC produced a significant reduction in PaCO2 but no significant change in RR, bicarbonate, or PaO2/FiO2 ratio, while IMV was avoided in 93.3 % of the cases. On the other hand, when comparing HFNC and NIV, Lee et al.59 reported no differences in the rate of OTI or 30-day mortality. Neither did they find significant differences in the values of pH, PaO2 and PaCO2. McKinstry et al,60 however, found that the application of HFNC to COPD patients reduced transcutaneous CO2 values and RR, with an increase in flow. CO2 reduction after implementing HFNC for 1h was also observed in a prospective, observational, analytical study.61 Sun et al62 studied patients with moderate hypercapnic respiratory failure. They compared the results of patients treated with HFNC or NIV. They found no significant differences in the rate of failure or mortality. They also report that the application time was longer in patients with HFNC. Kim and colleagues63 studied the application of HFNC in patients with ARF and hypercapnia. They used flow rates not higher than 50L/min and FiO2 <0.5 and, when compared to SO, they observed a reduction in the CO2 concentration. Finally, Bae et al64 evaluated the efficacy of HFNC compared with patients with ARF only and found that unadjusted hospital and ICU mortality was lower in patients with HFNC. When adjusting these data, no significant differences were found. Finally, a systematic review and meta-analysis that compared the use of HFNC versus NIV in hypercapnic respiratory failure, including patients with exacerbated COPD, cystic fibrosis, and other causes of hypoventilation, demonstrated that there are no significant differences between both respiratory therapies in terms of mortality (RR 0.86, CI95% 0.48–1.56), need for intubation (RR 0.80, CI95% 0.46–1.39), days of ICU stay, days of hospitalization, comfort or resolution of dyspnea, for which HFNC are an option for the treatment of this entity.65 Regarding the post-extubation management of patients with COPD, a randomized controlled study that compared the use of HFNC against NIV showed that there were no differences in terms of treatment failure (RR 5.8%, CI95% 23.8-12.4%, p 0.535), but HFNC were associated with greater comfort and fewer facial injuries (7 (6-8) vs 5 (4-7), p < 0.001) and (0 vs 9.6%, p 0.027) respectively.66
Immunosuppressed, transplant and cancer patients
The use of HFNC in immunosuppressed patients is controversial. There is evidence on the decrease in OTI in ARF and ARDS.67 Adequate tolerance, thermo-humidified flow, stable FiO2, as well as its non-invasive characteristics seem to be a reasonable option of treatment. Systematic reviews have demonstrated these advantages.68,69 These studies found a reduction in the rate of OTI, but not in the risk of mortality. Ricard et al.17 propose an explanation: “A possible explanation is that the underlying disease of the patients and/or the precipitating factor that leads to ARF in immunocompromised patients requires more recovery time. Consequently, these patients may have longer-lasting oxygen dependency and may require more invasive procedures. (…) For all these reasons, the nature of ventilatory support may not have such an impact in this particular setting”. Meta-analyses showed contradictory results in a heterogeneous population.70-72 The advancement of drug treatment and the advance directive of no-OTI are confusing. Advanced age and opportunistic infections, the main cause of ARF and mortality, are associated with a higher rate of OTI73 and HFNC would not have superiority in solid tumor and oncohematologic diseases.70 Its use in combination with NIV would not decrease mortality.74
For lung transplant patients, it was reported that absolute risk reduction of IMV with HFNC was 29.8%. Multivariate analysis showed that HFNC treatment was the only variable, on ICU admission, associated with a decreased risk of IMV. In addition, those patients who did not require IMV showed a higher survival rate and did not report adverse events associated with its use.23
Regarding cancer patients, a study reported that the use of HFNC-NIV resulted in lower mortality at 28 days; longer time from admission to OTI and higher, but not significant, number of ventilator-free days, compared with patients who used SO or NIV.30 In addition, after adjusting for Propensity Score, HFNC-NIV was independently associated with survival rates, while the OTI rate was similar for both groups.
Post-surgical period
In a systematic review and meta-analysis, Lu et al.75 observed that the use of HFNC after surgery was significantly associated with a reduction in hospital length of stay. These findings were not accompanied by a reduction in mortality, reintubation or pulmonary complications. Another systematic review and meta-analysis76 showed that HFNC was associated with a lower rate of reintubation and a reduction in IMV requirements; while another one77 showed that HFNC significantly reduce hypercapnia and OTI rate.
Regarding comfort, it has been shown that in cancer patients who have undergone esophagectomy, sore throat and/or nose in the group treated with HFNC was lower, while sputum production was greater and the total hospital length of stay was shorter. In addition, HFNC decreased systolic blood pressure, diastolic blood pressure and HR, increased PaO2 and SpO2 in the postoperative period.78
HFNC as orotracheal intubation adjuvant
Many preoxygenation technics have been used to reach a correct oxygen concentration during the pre orotracheal intubation. By using HFNC situations such as preoxygenation (mostly in patients at risk of desaturation), endoscopic procedures, difficult airways access or laryngeal surgeries, could be addressed. HFNC provides FiO2 100% and humidified and heated flow which is more comfortable and tolerable, despite using flows of 60L/min, or grater. However, conflicting results have been reported regarding apneic oxygenation79-81 and, because of that, the use of non-invasive ventilation combined with HFNC have been proposed.82 In a recent study which evaluates the oxygenation, using HFNC, during a rapid sequence induction showed less desaturation compared with a face mask preoxygenation.83 This results were different to those reported in patients undergoing neuromuscular blockades (in terms of maintaining PaO2 and PaCO2).84 Finally, HFNC seems to provide rapid and safe preoxygenation in obese people prior to general anesthesia,85 but in pregnant women seems to be not recommendable as preoxygenation tool.86
Monitoring
There are multiple monitoring strategies, most of them based on indexes or clinical parameters that are easy to assess.87 (Table 1).
Table 1.
Summary of some of the most representative studies on the HFNC effects
|
Study / Authors |
Objectives |
Patients (n) |
Results |
|
Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome Maggiore, SM, et al. |
To compare the effects of the Venturi mask and the nasal high-flow therapy on PaO2/FiO2 ratio after extubation. Secondary endpoints: to assess effects on patient discomfort, adverse events and clinical outcomes. |
105 patients with a PaO2/FiO2 ratio ≤ 300 immediately before extubation. Venturi mask (n 52) or NHF (n 53) |
Compared with the Venturi mask, HFNC results in better oxygenation for the same set FiO2 after extubation. Use of HFNC is associated with better comfort, fewer desaturations and interface displacements, and a lower reintubation rate. |
|
Optimum support by high-flow nasal cannula in acute hypoxemic respiratory failure: effects of increasing flow rates Mauri T et al. |
To assess the effects of HFNC delivered at increasing flow rate on inspiratory effort, WOB, minute ventilation, lung volumes, dynamic compliance and oxygenation in AHRF patients. |
A prospective randomized cross-over study with 17 patients |
In this cohort of patients with AHRF, an increasing HFNC flow rate progressively decreased inspiratory effort and improved lung aeration, dynamic compliance and oxygenation. |
|
Effects of High-Flow Nasal Cannula on the Work of Breathing in Patients Recovering from Acute Respiratory Failure Delorme M et al. |
To assess the effects of HFNC on indexes of respiratory effort (i.e., esophageal pressure variations, esophageal pressure-time product/min, and WOB/min) in adults. |
Randomized controlled crossover study in 12 patients |
HFNC, when set at 60 L/min, significantly reduces the indexes of respiratory effort in adult patients recovering from acute respiratory failure. This effect is associated with an improvement in respiratory mechanics. |
|
The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline Rochwerg B et al. |
Evidence-based recommendations regarding use of HFNC in various clinical settings. |
This clinical practice guideline synthesizes current best-evidence into four recommendations for HFNC use in patients with hypoxemic respiratory failure, following extubation, in the peri-intubation period, and postoperatively for bedside clinicians. |
|
|
Humidified high flow nasal cannula supportive therapy improves outcomes in lung transplant recipients readmitted to the intensive care unit because of acute respiratory failure Roca O et al |
The effectiveness of HFNC in lung transplant recipients readmitted to ICU because of ARF has not been determined yet. |
37 lung transplant recipients |
HFNC is feasible and safe and may decrease the need for MV in these patients readmitted to the ICU because of ARF. |
|
High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure Frat JP et al. |
Whether NIV should be administered in patients with hypoxemic ARF is debated. Therapy with HFNC through a nasal cannula may offer an alternative in patients with hypoxemia. |
A total of 310 patients were included in the analyses. |
In patients with nonhypercapnic hypoxemic ARF, treatment with HFNC, standard oxygen, or NIV did not result in significantly different intubation rates. There was a significant difference in favor of HFNC in 90-day mortality. |
|
A Randomized Controlled Trial of High-Flow Nasal Oxygen (Optiflow) as Part of an Enhanced Recovery Program After Lung Resection Surgery Ansari BM et al. |
To evaluate the routinely use of HFNC in patients undergoing thoracic surgery. |
59 patients were randomly assigned to either HFNC (n 28) or standard oxygen (n 31) |
Prophylactic HFNC, when incorporated into an enhanced recovery program, did not improve 6-minute walk test results but was associated with reduced length of hospital stay and improved satisfaction after lung resection, compared with standard oxygen. |
|
High-Flow Nasal Cannula Oxygen in Adults: An Evidence-based Assessment Drake MG |
To evaluate HFNC in terms of oxygen delivery and flow-dependent carbon dioxide clearance, WOB and inspiratory demand during respiratory distress. |
This review examines the evidence for HFNC oxygenation in adults, including a focus on the unique effects of high flow on respiratory physiology and keys for tailoring flow for specific clinical scenarios. |
|
|
ROX index as predictor of high flow nasal cannula therapy success in acute respiratory failure due to SARS-CoV-2 Ferrer S et al. |
To determine whether the ROX Index could predict HFNC therapy success in patients with ARF due to SARS-CoV-2 pneumonia. |
85 patients |
ROX index at 24 h with a cut-off point of 5.35 predicts HFNC success in patients with SARS-Cov-2-induced ARF. |
|
Effect of High-Flow Oxygen Therapy vs Conventional Oxygen Therapy on Invasive Mechanical Ventilation and Clinical Recovery in Patients with Severe COVID-19: A Randomized Clinical Trial Ospina-Tascón GA et al. |
To determine the effect of high-flow oxygen therapy through a nasal cannula compared with conventional oxygen therapy on need for endotracheal intubation and clinical recovery in severe COVID-19. |
Patients were randomly assigned to receive high-flow oxygen through a nasal cannula (n = 109) or conventional oxygen therapy (n = 111). |
Among patients with severe COVID-19, use of HFNC significantly decreased need for mechanical ventilation support and time to clinical recovery compared with conventional low-flow oxygen therapy. |
|
Effect of Noninvasive Respiratory Strategies on Intubation or Mortality Among Patients with Acute Hypoxemic Respiratory Failure and COVID-19 The RECOVERY-RS Randomized Clinical Trial Perkins GD et al |
What is the effect of initial noninvasive respiratory strategies using CPAP or HFNC, compared with an initial strategy of conventional oxygen therapy, on the risk of tracheal intubation or mortality among hospitalized adults with acute hypoxemic respiratory failure due to COVID-19? |
1273 patients |
An initial strategy of CPAP significantly reduced the risk of tracheal intubation or mortality compared with conventional oxygen therapy, but there was no significant difference between an initial strategy of HFNC compared with conventional oxygen therapy. The study may have been underpowered for the comparison of HFNC vs conventional oxygen therapy |
|
Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial Ehrmann S et al. |
To evaluate the efficacy of awake prone positioning to prevent intubation or death in patients with severe COVID-19 in a large-scale randomised trial. |
1126 patients were enrolled and randomly assigned to awake prone positioning (n=567) or standard care (n=559) |
Awake prone positioning of patients with hypoxaemic respiratory failure due to COVID-19 reduces the incidence of treatment failure and the need for intubation without any signal of harm. |
|
Impact of exposure time in awake prone positioning on clinical outcomes of patients with COVID-19-related acute respiratory failure treated with high-flow nasal oxygen: a multicenter cohort study Esperatti,M et al. |
To evaluated the effect of AW-PP on the risk of endotracheal intubation and in-hospital mortality in patients with COVID-19-related ARF treated with HFNO and analyzed the effects of different exposure times to AW-PP. |
580 patients were screened and 335 were included; 187 (56%) tolerated AW-PP for [median (p25-75)] 12 (9-16) h/day and 148 (44%) served as controls. |
In the study population, AW-PP for ≥ 6 h/day reduced the risk of endotracheal intubation, and exposure ≥ 8 h/d reduced the risk of hospital mortality. |
|
Nasal high flow eliminates CO2 from lower airways Bräunlich J et al |
To illustrate the wash-out mechanism to be effective even in the lower airways by means of an animal model |
Sheep lungs |
CO2 was decreased by HFNC in lower airways and in tracheal space. Changes in CO2 were flow dependent. There was also an increase in airway pressure in these settings. |
|
High-Flow Nasal Cannula Oxygen Therapy Can Be Effective for Patients in Acute Hypoxemic Respiratory Failure with Hypercapnia: a Retrospective, Propensity Score-Matched Cohort Study Bae SH et al |
To investigated the effectiveness of HFNC therapy for AHRF patients with hypercapnia compared to those without hypercapnia. |
862 patients (202 were included in the hypercapnic group) |
In acute hypoxemic respiratory failure with underlying conditions, HFNC therapy might be helpful for patients with hypercapnia. Large prospective and randomized controlled trials are required for firm conclusions. |
|
High flow nasal therapy in immunocompromised patients with acute respiratory failure: A systematic review and meta-analysis Cortegiani A et al |
Systematic review and meta-analysis in order to address the role of HFNC as compared to standard oxygen therapy in immunocompromised patients admitted to ICU with ARF |
872 patients |
No benefit of HFNC on mortality in immunocompromised patients with ARF. However, HFNC was associated with a lower intubation rate |
|
Effect of High-Flow Nasal Cannula Oxygen Therapy in Immunocompromised Subjects with Acute Respiratory Failure Kang H et al |
Meta-analysis to evaluate the effect of HFNC in immunocompromised patients with ARF versus conventional oxygen and NIV. |
2167 patients |
HFNC may be a feasible alternative to NIV, with lower intubation rates and no increased risk for ICU-acquired infections compared to standard oxygen therapy. However, HFNC did not appear to reduce mortality in immunocompromised subjects with ARF compared with other noninvasive therapies |
|
The Effect of High-Flow Nasal Oxygen Therapy on Postoperative Pulmonary Complications and Hospital Length of Stay in Postoperative Patients: A Systematic Review and Meta-Analysis Lu et al |
To evaluate the effect of HFNC on hospital length of stay and postoperative pulmonary complications in adult postoperative patients. |
2568 patients |
Among adult postoperative patients, HFNO therapy significantly reduces hospital length of stay. |
|
High-Flow Nasal Cannula in the Immediate Postoperative Period: A Systematic Review and Meta-analysis Chaudhuri D et al |
To evaluate data examining routine HFNC use in the immediate postoperative period. |
2,201 patients |
With evidence of moderate certainty, prophylactic HFNC reduces reintubation and escalation of respiratory support compared with standard oxygen in the immediate postoperative period after cardiothoracic surgery. |
|
Apneic oxygenation is associated with a reduction in the incidence of hypoxemia during the RSI of patients with intracranial hemorrhage in the emergency department Sakles JC et al |
To evaluate apneic oxygenation may be able to reduce the occurrence of oxygen desaturation during the emergent intubation |
127 patients. |
Patients who received apneic oxygenation were seven times less likely to have an oxygen saturation <90 % during the intubation compared to patients who did not receive apneic oxygenation |
|
First Pass Success Without Hypoxemia Is Increased with the Use of Apneic Oxygenation During Rapid Sequence Intubation in the Emergency Department Sakles JC et al |
To determine the effect of apneic oxygenation on first pass success without hypoxemia in adult patients undergoing rapid sequence intubation (RSI) in the emergency department |
635 patients |
The results suggest that the use of apneic oxygenation has the potential to increase the safety of RSI in the ED by reducing the number of intubation attempts and the incidence of hypoxemia. |
|
Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: The utility of the ROX index Roca J et al |
To describe early predictors and to develop a prediction tool that accurately identifies the need for mechanical ventilation in pneumonia patients with hypoxemic ARF treated with HFNC |
157 patients |
In patients with ARF and pneumonia, the ROX index can identify patients at low risk for HFNC failure in whom therapy can be continued after 12 hours. |
The ROX index (calculated as [SpO2/FiO2]/RR) was used to predict the success of therapy 12h after starting HFNC treatment, with 4.88 being the reference value.88 A subsequent evaluation showed similar values.89 Other authors proposed a modification to this index.90 In addition, including HR showed a significant association of a value >8.00 at 10h after starting HFNC therapy and a lower risk of failure.86 Cut-off points for this index and different evaluation times have been reported, but to date there is no consensus on the exact time at which it should be evaluated.36,91,92 An index including HR and SpO2 has been proposed to predict failure and OTI requirement, which showed greater precision, in patients with moderate hypercapnia.53 Sustained tachypnea has been associated with respiratory muscle fatigue in critically ill patients and with the need for IMV and, by itself, is a factor associated with HFNC failure in patients with COVID-19.93
Due to its simple and non-invasive measurement, the ROX index is the most studied and widespread prediction score; however, some limitations and controversies about the actual cut-off point or when it should be evaluated should be considered.6,95 Finally, to determine the risk of intubation in patients with HFNC, WOB assess94 and the capacity of the HACOR score has been proposed, reporting a moderate predictive capacity92 No index showed superiority over the others.
Potential risks associated with the use of CNAF
ARDS patients with spontaneous ventilation could worsen their clinical status and be at risk of P-SILI due to excessive inspiratory effort.13 Furthermore, HFNC can mask a deterioration in the ventilation/perfusion ratio and the presence of a hidden Haldane effect secondary to anatomical dead space washout and CO2 reduction. This situation could trigger silent hypoxemia and a false perception of clinical improvement. As postulated, patients could show respiratory muscle fatigue, cardiac dysfunction and organ failure and trigger worse outcomes.18
One of the main fears is the delay of intubation. A recently published study showed that late intubation is associated with increased mortality,96 while another one97 showed a mortality rate of 27.3% in patients with HFNC, who required IMV later. Finally, a study98 reported lower mortality in those patients who were intubated early compared to those who underwent OTI later.
Conclusion
HFNC has become popular in recent years, being one of the main treatment strategies for AHRF. The results reported in other clinical scenarios have shown variations depending on the study design and population, determining that patients must be carefully selected. Its monitoring, bedside and non-invasive, seems simple but must be interpreted considering the underlying pathophysiology of the disease. The evidence seems to support that its correct use can be associated with lower morbidity and mortality.
Declarations
Ethical Approval: not applicable.
Funding: none.
Conflict of interest: the authors have no relevant financial or non-financial interests to disclose
Author’s contributions: AG contributed to the study conception and design. AG, JMNS, CG, ORPN, AD: Material preparation, data collection and analysis. AG: first draft of the manuscript. AG, AD, CG, EZL, ORPN, JMNS: commented on previous versions of the manuscript. AG, AD, CG, EZL, ORPN, JMNS: read and approved the final manuscript.
Availability of data and materials: all the studies used in this systematic review are available, in their entirety, on Medline (Pubmed)
The Editor in Chief, Dr. Carlos Luna, did the peer review follow- up and approved this article.
References